VANRAFIA® Results

VANRAFIA was studied in adults with IgAN as a once-daily add-on to current blood pressure medication*
Who was studied?
A total of 340 adults with biopsy-proven IgAN who:
had total urine protein ≥1 g/day
were on a stable dose of maximally tolerated blood pressure medication, like ACEi or ARB
The initial results were based on the first 270 people who reached the 9-month visit who:
had an average age of 45 years (19 to 77 years)
were 59% male and 41% female, 57% Asian, 36% White, and 2% Black or African American
What was studied?
Of the 270 adults who reached the 9-month visit:
135 adults received 0.75 mg of VANRAFIA once daily in addition to blood pressure medication*
compared to
135 adults who received placebo (sugar pill) in addition to blood pressure medication*
How was the study done?
Changes in protein in urine (proteinuria) were assessed using the UPCR test in urine samples collected over 24 hours at study initiation and through 9 months of treatment.
*Participants were on a stable and maximally tolerated dose of a blood pressure medication, like ACEi or ARB.
Track your kidney health with this helpful tool
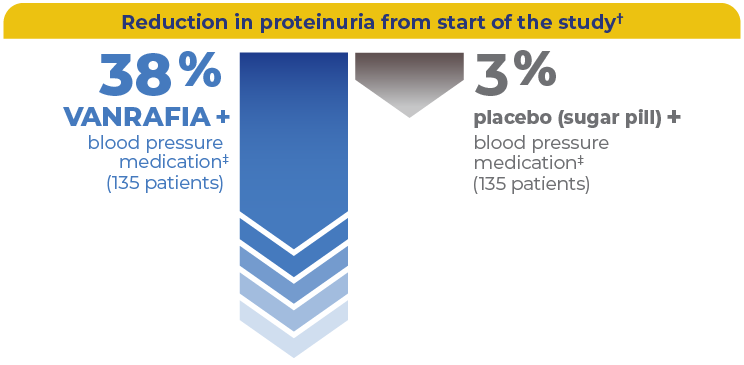
Results with VANRAFIA
In adults with primary IgAN at risk of their disease getting worse quickly
VANRAFIA substantially reduced proteinuria at 9 months
As an add-on to a blood pressure medication‡
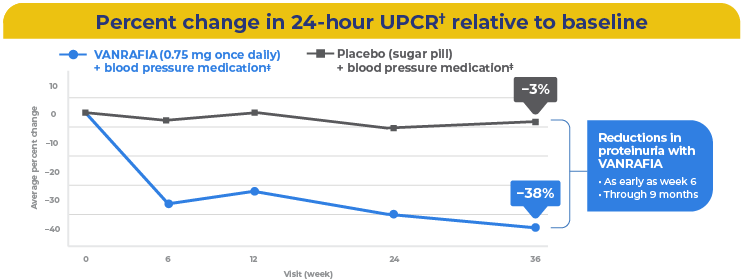
Rapid and sustained proteinuria reduction with VANRAFIA through 9 months
†This study assessed urine protein levels using samples taken over 24 hours.
‡Participants were on a stable and maximally tolerated dose of a blood pressure medication, like ACEi or ARB.